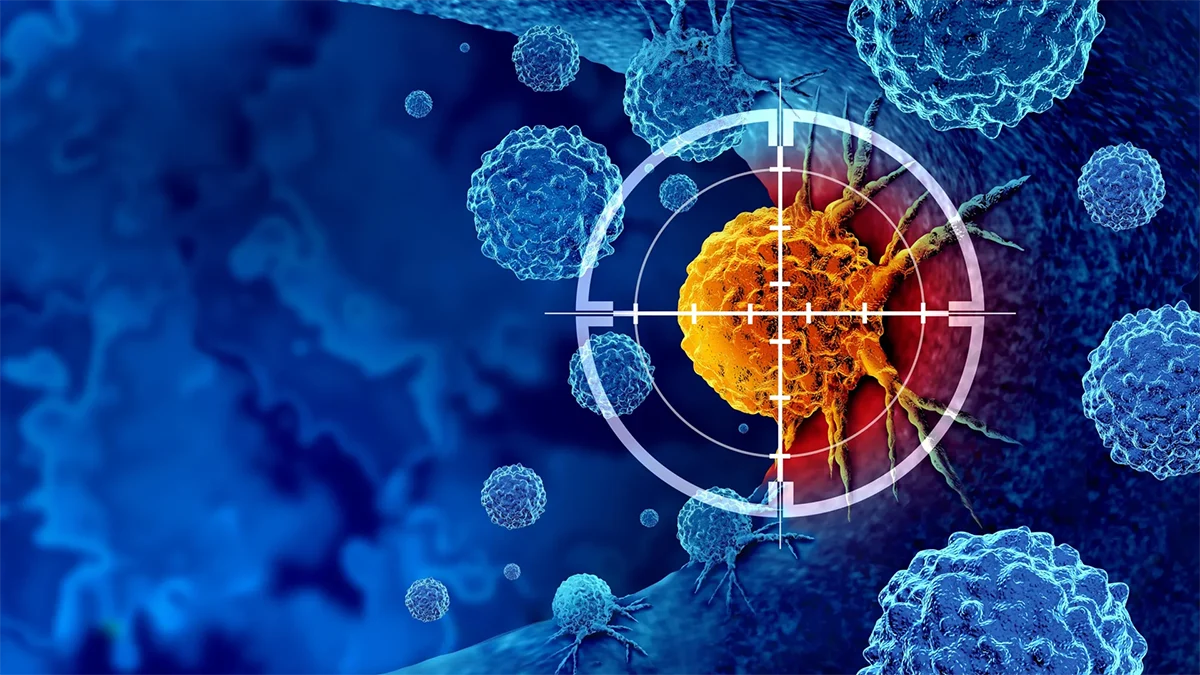
Researchers have identified an experimental antibody that shows promise against triple-negative breast cancer (TNBC), one of the deadliest and most treatment-resistant forms of the disease. This type of cancer grows rapidly, spreads early and lacks the hormone receptors that many targeted drugs rely on, making effective treatments scarce.
The new research, published in Breast Cancer Research, describes how scientists engineered a humanized monoclonal antibody to attack multiple survival mechanisms used by TNBC cells. The experimental antibody not only slowed tumor growth, but also reduced lung metastases and revived immune cells that normally defend the body against cancer.
🧬 Targeting a Key Cancer-Promoting Protein
The preclinical study focused on secreted frizzled-related protein 2 (SFRP2), a protein that helps tumors thrive by promoting new blood vessel formation, preventing cancer cells from dying, and weakening immune responses. The researchers found that SFRP2 appears not only in TNBC cells but also in immune cells within the tumor environment.
By attaching the antibody directly to SFRP2, the treatment effectively blocked these cancer-promoting influences. The antibody pushed immune cells called macrophages toward a cancer-fighting state while boosting the activity of T-cells, another critical part of the body’s immune defense.
🧬 Immune System Reprogrammed Around Tumors
In the study, the antibody influenced both macrophages and T-cells to become more active against tumors. Macrophages normally swing between two states: one that suppresses immune activity and one that activates it. In TNBC, the suppressive state often dominates and blunts immune attack.
However, antibody treatment shifted macrophages toward a more aggressive, immune-activating status without causing the toxic effects that direct immune signaling molecules can produce. Meanwhile, nearby T-cells regained activity, indicating the potential for stronger overall immune responses against TNBC.
🩸 Reduced Metastases in Preclinical Models
In tests on TNBC models that had advanced disease, the antibody also led to significantly fewer lung metastases. Lung metastasis indicates that cancer spread through the bloodstream, contributing to worse outcomes in patients. The fact that the antibody lowered metastatic spread in these models suggests promise beyond slowing primary tumor growth.
Importantly, the antibody behaved differently from many traditional chemotherapies. While standard drugs often attack both cancerous and healthy cells, this antibody accumulated mainly in tumor tissue. That meant fewer off-target effects in healthy organs or cells, an encouraging safety signal in early research.
🔬 Effectiveness Against Chemotherapy-Resistant Cells
Another major challenge in TNBC is resistance to chemotherapy. In the study, researchers tested the antibody against TNBC cells that had stopped responding to a common chemotherapy drug. Even in those resistant cells, the antibody still triggered substantial cancer cell death, suggesting it might offer a new approach where standard treatments fall short.
🔮 Next Steps in Research
The findings remain early and come from preclinical studies. However, the antibody’s ability to slow tumor growth and restore immune responses highlights its potential.
Researchers have licensed the antibody to a biotechnology company. The company plans to pursue human clinical trials in the future, supported by regulatory designations for difficult-to-treat cancers.

0 Comments